Methanol And Cyclohexane Phase Diagram With Upper Critical T
Solved 2- the liquid-phase reaction of methanol (a) and Calculated phase diagrams for binary blends of methanol and co 2 at Solved 4. a phase diagram for methanol-water is provided on
Solved Consider the phase diagram for methanol provided | Chegg.com
The coexisting liquid phases for the system methanol Phase changes. Solved:a mixture of cyclohexane and cyclopentane is to be separated by
Critical temperature of methanol
Miscibility gap of heptane-methanol mixturePhase diagram of a supercritical fluid. 1) exchange methanol with Make the pt diagram for methanol. show and identifySolved 2. 7. [15%) using following temperature-composition.
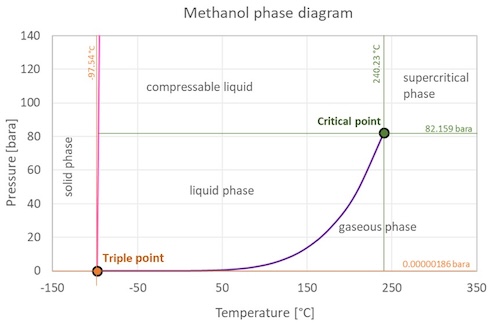
Methanol phase diagramIsothermal vapor-liquid phase diagram of cyclohexane + cyclohexanone at Solid, liquid and gaseous methanol can only coexist at pressure abovePt project of methanol+cyclohexane mixture, showing the saturation.

3. below is a temperature/composition diagram for a
(a) phase diagram of methanol shows route 1 employed in a conventional4. a phase diagram for methanol-water is provided on Phase diagram of ethanolSolved: methanol water liquid vapor phase diagram.
High pressure phase equilibrium for methanol-water solutionsPhase diagrams methanol mixtures The phase diagram of cyclohexane–methanol: a challenge in chemicalCyclohexane phase vapor liquid isothermal cyclohexanone experimental.

Phase diagram of methanol based on anomalies of the static dielectric
Water/methanol phase diagramLib.meos.cyclohexane — pychemqt 0.1 documentation The phase diagram of methanol. the critical point (t c = 239.5 °c, p cPt project of methanol+cyclohexane mixture, showing the saturation.
Methanol criticalMethylcyclohexane methanol Methanol water phase diagram pressure solutions data al equilibrium vuillard haghighi adapted kargel atmospheric et 2009Methanol dielectric anomalies constant.

Solved ok 1 tpa temperature 50k 100 k 150 k 200 k 250k 300 k
Methylcyclohexane methanol(a) the phase diagram analysis of n-hexane-methanol-water system and Solved consider a mixture of methanol (1) and cyclohexaneSolved consider the phase diagram for methanol provided.
Methanol employed conventional temperature supercriticalSolved: explain the fact that, although hemiacetal formation between .